AMINO ACID REQUIREMENTS IN RUMINANTS
Considering that each protein synthesized by the animal organism is genetically determined and possesses a unique and unchanging amino acid (AA) composition, the requirement varies for each AA. Therefore, maintaining a balance of AAs in the diet of ruminants is essential (SCHWAB; WHITEHOUSE, 2021).
Out of the 20 AAs present in proteins, 9 are classified as nutritionally essential (indispensable), and one (arginine [Arg]) is considered conditionally essential. Essential AAs cannot be synthesized in the body or are inadequately produced endogenously and must be obtained through the diet. The essential AAs include: 1) histidine (His); 2) isoleucine (Ile); 3) leucine (Leu); 4) lysine (Lys); 5) methionine (Met); 6) phenylalanine (Phe); 7) threonine (Thr); 8) tryptophan (Trp); and 9) valine (Val) (NASEM 2021).
 In the case of Arg, even though there is a significant level of de novo synthesis, for high-producing cows, synthesis is insufficient to meet the demand. Consequently, Arg is traditionally considered an essential AA for ruminants. The other 10 AAs are classified as nutritionally non-essential due to their ability to be synthesized de novo from nitrogen sources in sufficient quantities, either from excess essential AAs or from other non-essential AAs (NASEM, 2021; SCHWAB; WHITEHOUSE, 2021).
In the case of Arg, even though there is a significant level of de novo synthesis, for high-producing cows, synthesis is insufficient to meet the demand. Consequently, Arg is traditionally considered an essential AA for ruminants. The other 10 AAs are classified as nutritionally non-essential due to their ability to be synthesized de novo from nitrogen sources in sufficient quantities, either from excess essential AAs or from other non-essential AAs (NASEM, 2021; SCHWAB; WHITEHOUSE, 2021).
For ruminants, AA absorption occurs through three main pathways:
- Microbial Proteins (MicP) synthesized in the rumen,
- Undegraded Dietary Protein in the Rumen (UDR), and, to a lesser extent,
- Endogenous proteins (SCHWAB; WHITEHOUSE, 2021).

Scientific evidence suggests that microbial protein generally accounts for over 50% of the flow of crude protein (CP) to the small intestine (SCHWAB; BRODERICK, 2017).
For a long time, it was assumed that MicP (synthesized in the rumen) combined with a portion of UDR (dietary protein escaping ruminal degradation) would fulfill all AA requirements. This assumption was likely accurate for low-producing cows used in initial experiments (considering approximately 2,300 kg/year average milk production in the United States). However, with the increase in average production, this assumption needed reassessment (SCHWAB; BRODERICK, 2017).
In a classic experiment using 15N-labeled ammonium sulfate for lactating cows, it was observed that 15 hours after feeding, all amino acids in milk proteins (separated by fractionation after hydrolysis) were labeled. This demonstrated that MicP could provide all the necessary AAs for milk protein synthesis. However, the labeling level differed among AAs, with higher labeling for non-essential AAs compared to essential AAs. It is assumed that there was a higher degree of labeling for non-essential AAs because these can be synthesized by the ruminant organism (especially in the liver from ammonia) and not solely from microbial protein, as is the case with essential AAs (VIRTANEN, 1966).
Considering the need to quantify microbial protein synthesis to meet the requirements of lactating cows, the same authors conducted a study by feeding lactating cows diets with urea nitrogen as the only nitrogen source, without protein sources. Despite no negative impact on milk production or milk protein composition under the experimental conditions tested, there was a lower concentration of essential AAs in the blood plasma of lactating cows fed diets with all protein coming from non-nitrogen-protected (NNP) sources compared to cows in the control group (VIRTANEN, 1966).
Furthermore, it is worth noting that the rapid hydrolysis of urea into ammonia in the rumen may exceed the capacity of ruminal microbiota utilization. When there is an excess of nitrogen relative to energy in the rumen, unused ammonia is absorbed by the rumen epithelium, taken up by the liver via the portal system, and converted into urea for detoxification. Urea produced from hepatic detoxification circulates in the blood and can be excreted in urine, recycled to the rumen, or diffused in milk, where it can be detected by measuring urea nitrogen in milk (HEIDARI et al., 2022; SCHWAB; BRODERICK, 2017). Therefore, for the correct supply of AA requirements in the diet of dairy cows, a proper balance between UDR and NDR sources must be considered.
Considering the main absorption pathways, NASEM (2021) recommendations for each essential AA are calculated using a factorial approach, representing a quantitative evaluation based on the sum needed to fulfill the designated metabolic function of each AA. For estimating post-ruminal flow of essential AAs, a factorial method based on predicting microbial protein, UDR, and endogenous duodenal crude protein is used. Individual AA flow considers the net supply of AAs and is calculated from the corresponding AA composition and digestibility of UDR and crude microbial protein.
In the case of endogenous duodenal proteins, although they contribute to duodenal protein flow, as they are synthesized by the organism itself and therefore do not constitute a new net supply of AAs, their contribution to metabolizable AA flow is not considered (NASEM, 2021).
Individual recommendations are provided for all essential AAs, with the sole exception of Arg. The approach used for AA recommendations follows that used for metabolizable protein, and a combined efficiency goal is used for each AA, assuming that energy requirements are adequately met (NASEM, 2021). Current NASEM (2021) recommendations use a multifactorial nonlinear function with additive responses to independent variables, unlike the old linear representation model of the relationship between metabolizable protein or AA supply and milk protein production. For this reason, it is not possible to define a fixed requirement for any of the determining variables. Instead, an infinite combination of variables can be associated with a particular production level. Therefore, it is crucial to establish biological guidelines and limits to be followed. Firstly, it is necessary to establish and quantify the direct net requirement for the supply of essential AAs, whether through protein secretion or protein accretion, indicating the essential AAs removed from the net base that must be replenished promptly. Then, an efficiency of utilizing the source of essential AAs to meet each function is assigned. Recommendations are then calculated from the net protein requirement divided by the utilization efficiency.
In summary, recommendations for metabolizable protein should be used as a guideline only, with greater emphasis on balancing essential AAs individually. Diets that provide sufficient amounts of metabolizable protein may be deficient in one or more essential AAs, while diets with seemingly insufficient amounts of metabolizable protein may be balanced for essential AAs, and individual AA balance presents a better approach to milk protein production (NASEM, 2021).
Table 1. Example of Adequate Supply of Essential Amino Acids (EAAs) for a Lactating Cow with Graduated Milk Protein Production (values expressed as % of protein) and Composition of EAAs in Feeds and Rumen Microbiota
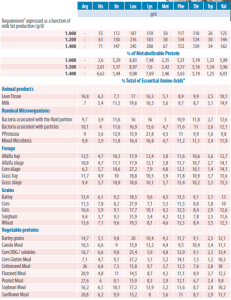
¹Example of Adequate EAA supplies for an empty multiparous cow (650kg BW), consuming 26kg/d of a diet with 34% NDF with graduated milk protein (values expressed as % of protein). Adapted from NRC 2021; ²Composition of EAAs in feeds and rumen microbiota. Adapted from (SCHWAB; WHITEHOUSE, 2021)
SUPPLEMENTATION WITH ESSENTIAL AMINO ACIDS
Lactating cows with high levels of milk production have increased requirements, which may need the use of synthetic essential amino acids (rumen-protected) for a complete amino acid balance (ALVES, 2004). Through supplementation, the absorption of AAs in the post-rumen is expected, requiring the escape of free AAs based on the administered dose. Studies suggest that an increase in the dose of AAs infused into the rumen leads to a substantial decrease in ruminal degradation (ALVES, 2004; COTTLE; VELLE, 1989; SCHWAB; BRODERICK, 2017).
Additionally, supplementation with protected AAs results in the escape of a significant amount of AAs from the rumen unchanged (ALVES, 2004; COTTLE; VELLE, 1989). However, the commercial production of high-quality rumen-protected AA supplements is challenging (SCHWAB; BRODERICK, 2017).
Among the essential amino acids whose supplementation is recommended in ruminants, lysine and methionine are the most limiting and, therefore, show the greatest productivity benefit from supplementation (SCHWAB; BRODERICK, 2017).
Various approaches have been evaluated to physically protect lysine and methionine from the action of ruminal microorganisms (e.g., protection by lipids, often in combination with inorganic materials and carbohydrates as stabilizing agents). The most effective approach has been through a post-ruminal passage system and intestinal release associated with pH differences between the rumen and abomasum, independent of enzymatic function. The method involves coating the AA surface with polymers resistant to enzymatic degradation but containing synthetic compounds sensitive to pH, insoluble in the ruminal environment, and highly soluble in the more acidic abomasum pH. Compared to other methods, polymer-protected AAs have a higher intestinal release coefficient (SCHWAB; WHITEHOUSE, 2021).
Derivatives of methionine and lysine are also an alternative for supplying these amino acids to ruminants. These refer to free AA analogs to which a chemical blocker has been added to the alpha-amino group or the acyl group has been replaced by a non-nitrogenous group.
Studies evaluating the methionine analog 2-hydroxy-4-(methylthio) butanoic acid (HMTBa), the most studied analog for ruminants, indicate greater resistance of free HMTBa to ruminal degradation compared to free methionine. Furthermore, free HMTBa has higher ruminal absorption and is converted into methionine in tissues. However, no increase in methionine concentrations in blood plasma or milk protein concentrations in methionine-deficient cows supplemented with HMTBa was observed, and for this reason, the use of protected AAs remains the most effective alternative.
Supplementation with rumen-protected AAs requires, in addition to analyzing the content and cost of supplements, knowledge of AA absorption when provided according to the herd’s feeding program (SCHWAB; BRODERICK, 2017). Knowing the ruminal degradability value is crucial for planning the supplementation of diets with synthetic AAs. Considering this, estimates of bioavailability for supplementation with synthetic AAs must be accurate and reliable (SCHWAB; BRODERICK, 2017). Analyzing the concentration of free AAs in the rumen, obtained from the proteolysis of diet protein sources, low observed concentrations indicate rapid degradation by ruminal microbiota. AAs obtained by proteolysis are incorporated by microorganisms for multiplication or are deaminated, producing ammonia nitrogen (ALVES, 2004). In this sense, formulating lower-cost diets using synthetic AAs to meet the demands of high-production cows requires information on the bioavailability of AAs (SCHWAB; BRODERICK, 2017).
The stability of rumen-protected AAs should also be considered when mixed with other feeds in total ration supply, especially when there is high moisture content (SCHWAB; BRODERICK, 2017).
Ji et al. (2016) evaluated the interaction between diet ingredients and rumen-protected AA supplements during total ration preparation in two correlated experiments. The first experiment aimed to assess the effect of ration moisture content on lysine supplementation, and the second experiment aimed to determine if mechanical mixing of the total ration alters lysine release in the rumen. Six different rumen-protected lysine supplements were evaluated in both experiments. There was a significant increase in lysine ruminal release in one supplement and a tendency to increase in another after mechanical mixing of the total ration. For all products tested, the exposure time to the ruminal environment altered lysine release, and a significant interaction between ruminal incubation hours and total ration mechanical mixing was observed for two products. Additionally, exposure time of the supplements to the diet significantly increased lysine release for all evaluated products, with one product showing a significant interaction between exposure time and dry matter content, resulting in higher lysine release in diets with lower dry matter content. The data suggest that the farm feeding program can alter the effectiveness of rumen-protected AAs, emphasizing the difficulty of developing stable AA supplements for ruminant nutrition. Furthermore, the results reinforce that the evaluation of the bioavailability of rumen-protected AA supplements should also involve interaction with the total ration and the farm feeding program (JI et al., 2016; SCHWAB; WHITEHOUSE, 2021).
EFFECTS OF AAs SUPPLEMENTATION
Supplementing essential amino acids in the diet of lactating cows aims to meet the demand and improve the amino acid balance when dietary protein sources are insufficient (SCHWAB; BRODERICK, 2017). Effects on milk production, milk protein synthesis (BRAKE et al., 2013), weight gain, and the immune response of dairy cows are observed through supplementation (ABREU et al., 2023).
We share a series of studies conducted to evaluate the correct supplementation of AAs in dairy cows and its effects on productive parameters.
1 Brake et al. (2013) evaluated the inclusion of a product containing methionine mixed with soy lecithin and added to mechanically extracted soybean meal (meSBM-Met) in milk production and composition and plasma methionine concentration of lactating cows. The meSBM-Met was compared to rumen-protected methionine supplementation (RPMet) and the control in a Latin square design experiment (5×5; 14 days per treatment, 10 adaptation days, and collections on the last 4 days). 25 multiparous Holstein cows (630 ± 54 kg body weight, 44.9 ± 7.0 kg milk/d, and 87 ± 28 DIM) were used.
Cows were blocked by DIM and distributed into five groups:
- a) control;
- b) low inclusion of meSBM-Met;
- c) high inclusion of meSBM-Met;
- d) low inclusion of RPMet; and
- e) high inclusion of RPMet, according to table 2.
Individual milk production per animal was recorded, and milk samples were collected for composition on the last 4 days of each period. Urine and feces samples were collected twice a day on the last 4 days of each period to determine N balance, and plasma samples were collected for AA concentration determination on day 14 of each period. In vitro methionine degradation determination was also performed for each treatment using ruminal fluid samples from two cows. Plasma methionine concentration increased linearly with higher RPMet-supplemented amounts, but not when methionine was provided by meSBM-Met. In vitro methionine degradation results suggest extensive ruminal degradation of methionine with meSBM-Met supplementation, leading to insufficient metabolizable methionine supply. A higher true milk protein concentration was observed when methionine was supplemented as RPMet compared to meSBM-Met. Despite this, N intake and retention did not differ between treatments, contributing to similar amounts of N excreted in urine and feces. No difference was observed between diets in dry matter intake, apparent digestibility, and average milk production. In this regard, milk protein content was the production parameter that responded best to the increased supply of metabolizable methionine, making it the most significant effect of supplementation.
2 Similar to the findings of Brake et al. (2013), an increase in milk protein production was also observed by Socha et al. (2005) in a study evaluating methionine and methionine and lysine supplementation in rumen-protected form in postpartum and early lactation cows. The study was conducted in a randomized block design, with 84 Holstein cows blocked by calving date and randomly assigned to three diets: a) basal diet (control); b) basal diet supplemented with 10.5 g of methionine (Smartamine M); and c) basal diet supplemented with 10.2 g of methionine and 16.0 g of lysine (Smartamine ML). In addition to the increase in milk protein production, an increase in energy-corrected milk production and milk fat was also observed in the diet with methionine and lysine supplementation. It is important to note, however, that in both studies, essential amino acid supplementation occurred in basal diets that met protein requirements, and supplementation might not necessarily have the same effect in low-protein diets.
3 To understand the effect of limiting essential amino acid supplementation in low-protein diets, Van den Bossche et al. (2023) assessed the supplementation of methionine, lysine, and histidine in lactating cows’ nutrition. The experiment involved 39 lactating Holstein cows (22 primiparous, 17 multiparous; 638 ± 73.2 kg body weight, average milk production of 32.7 ± 5.75 kg, DIM of 116 ± 29.3). The experimental design consisted of a 4-week depletion period (control), followed by a 7-week treatment period, and again a control (a 5-week crossover period). In the first phase, all cows received a control diet (hypoproteic; 14.5% CP), and after this period, cows were blocked by milk production, number of lactations, and plasma histidine concentration (collected along with milk samples on the last day of the pre-experimental period) and randomly assigned to three different diets during the experimental period (7 weeks): a) control diet (n = 13); b) control diet supplemented with protected methionine and lysine (MetLys, n = 13); c) control diet supplemented with protected methionine, lysine, and histidine (MetLysHis, n = 13). No effect of supplementation was observed on dry matter intake or productive performance, despite additional supply of dMet, dLys, and dHis evident in the bloodstream. The authors point out that the observed result may possibly be related to an overestimation of dietary AA supply at the beginning of the experiment, leading to methionine and lysine deficiency despite supplementation affecting the efficiency of AA utilization for milk protein production and synthesis.
Table 2. Composition of diets provided to cows (% of DM) in the study by Brake et al. (2013)
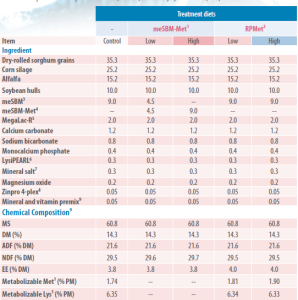
¹Mechanically extracted soybean meal with added Met during manufacturing. Based on the manufacturing process, low = 3.8 g Met/d total, and high = 7.6 g Met/d total. Based on product analysis, low = 2.7 g total Met/d, and high = 5.3 g Met/d total; ² Mechanically extracted soybean meal with soy lecithins added during manufacturing. Contained by analysis (% DM): Asp, 5.05; Thr, 1.71; Ser, 2.37; Glu, 8.02; Gly, 1.89; Ala, 1.94; Val, 1.97; Ile, 2.23; Leu, 3.41; Tyr, 1.38; Phe, 2.25; His, 1.15; Lys, 2.59; Arg, 2.73; Cys, 0.62; Met not detected; ³ Mechanically extracted soybean meal with Met mixed with soy lecithins and added during manufacturing, containing by analysis (% DM): Asp, 4.95; Thr, 1.70; Ser, 2.32; Glu, 7.88; Gly, 1.87; Ala, 1.92; Val, 1.97; Ile, 2.22; Leu, 3.38; Tyr, 1.29; Phe, 2.24; His, 1.12; Lys, 2.47; Arg, 2.67; Cys, 0.59; Met free, 0.23; ⁵ Calcium soap of long-chain fatty acids (Church and Dwight Co., Princeton, NJ); ⁶ Rumen-protected Lys providing 16.2 g/d of metabolizable Lys (Kemin Industries Inc.; product assumed to contain 21% metabolizable Lys); ⁷ Containing 96% NaCl, 0.35% Zn, 0.2% Fe, 0.2% Mn, 0.03% Cu, 0.007% I, and 0.005% Co; ⁸ Containing 2.58% Zn as Zn-Met; 1.43% Mn as Mn-Met; 0.90% Cu as Cu-Lys; 0.18% Co as Co-glucoheptonate (Zinpro Corp., Eden Prairie, MN); ⁹ Providing in diets (based on DM) 3,300 IU vitamin A/kg, 2,250 IU vitamin D/kg, 35 IU vitamin E/kg, and 0.06 mg Se/kg; ¹⁰ Calculated based on analyses of samples from the basal mixture, meSBM, and meSBM-Met; ¹¹ Estimated from the NRC model (2001). Values for diets containing meSBM-Met were not calculated because ruminal escape and intestinal digestion of escaped Met were unknown.
In addition to productive parameters, the supplementation of essential amino acids (AA) also has an effect on the immune response and, consequently, on the health of dairy cows.
4 In order to evaluate the effect of rumen-protected Met and Lys supplementation in a low forage diet on milk production, composition, and mammary gland health, Abreu et al. (2023) conducted an experiment on a commercial herd of 4800 lactating cows (11,200 kg/cow [305-day lactation]). Three hundred and fourteen mid-lactation multiparous cows were randomly allocated to two groups: a) control (107 g of dried distillers’ grains [DDG]); n = 152; b) RPML (107g of DDG supplemented with Smartamine ML [44% L-Lys; 15% D-Met] and 2.2 g of Smartamine M [n = 75% D-Met]); n = 162. Blood samples from 22 randomly selected cows were collected to determine the concentration of AA, minerals, and urea nitrogen in plasma. Additionally, daily milk production (per cow) was measured, and milk samples were collected for composition evaluation (total solids, fat, true protein, lactose, and milk urea nitrogen) and somatic cell count (SCC). A higher plasma concentration of Met and Lys in the RPML group was observed when expressed as a proportion of essential AA and total AA. There was no observed effect of supplementation on milk production or composition; however, there was an effect on mammary gland health. Cows in the RPML group had 0.39 times less risk of clinical mastitis than cows in the control group, indicating the impact of essential AA supplementation on the immune system and inflammatory response, possibly indicating a relationship between Met and Lys in the innate immune system response to intramammary infections.
CONCLUSIONS
Meeting the requirements of essential AA is fundamental for the optimal productive performance of cattle. Therefore, it is crucial to balance the diet appropriately to meet the requirements of Met, Lys, and His. Properly supplying the requirements of essential AA in the diet of dairy cows should consider the appropriate balance between RUP and RUPN sources. Furthermore, supplementation with rumen-protected synthetic AA can be an alternative for diets of high-production cows. However, technologies for supplement manufacturing have been a challenge, and for the inclusion of these products in the diet, attention must be paid to the stability and bioavailability of AA.
The results of adequate essential AA supplementation include an increase in milk protein production and improvement in the cows’ immune system response capacity.
References available upon request.
You may also like to read: “The Role of Rumen-Protected Amino Acids and Choline”