Definition & classification

Processed animal proteins (PAPs) are ingredients derived exclusively from category 3 animal by-products (ABP’s)

![]() PAPs include any healthy animal part which is not used to produce food for human consumption.
PAPs include any healthy animal part which is not used to produce food for human consumption.
The use of animal protein in feed from non-ruminant animals was reauthorized on 17 August 2021. With the the publication of a Regulation that relaxed the ban on proteins from animal origin in feed (Regulation (EU) 2021/1372)/ after 20 years of prohibition (Regulation (EU) 999/2001).
![]() In aquaculture, this reauthorization has been in force since 2013.
In aquaculture, this reauthorization has been in force since 2013.
![]() In ruminants, the use of this type of ingredient is still prohibited.
In ruminants, the use of this type of ingredient is still prohibited.
The new regulation establishes strict conditions under which these ingredients can be used in pig and poultry feed. Aiming to:
- Ensure compliance with the ban on intraspecific recycling (i.e. avoid cannibalism)
- Avoid cross-contamination and favor traceability
- Facilitate official controls on feed

PAPs are ingredients with a high protein content (45-70%) of high biological value, which can be superior to other protein sources such as:
- Soybean meal
- Rapeseed meal
- And legumes
In addition, they are a source of local protein, produced in Europe, which gives it added value in terms of sustainability and sovereignty.
The “Catalogue of raw materials” (Regulation (EU) 68/2013) classifies processed animal protein in section “9. Products from terrestrial animals and their derived products” (Table 1).

It is mandatory to declare protein, fat and ash contents.
Procurement process
![]() These ingredients must be treated in accordance with Section 1 Chapter II, Annex X of Regulation (EU) No 142/2011 during their procurement process.
These ingredients must be treated in accordance with Section 1 Chapter II, Annex X of Regulation (EU) No 142/2011 during their procurement process.
In general, these products must undergo procedures that combine temperature and pressure for a certain time to achieve adequate sanitation of the product.
Chemical composition and nutritional value
By their definition, it is expected that new PAPs present high variability due to their origin. Considering that they may contain any part of the animal.
⇒ There is still very little information regarding this variability and its possible classification and composition.
Several studies conducted with broilers at Wageningen University (Krimpen et al., 2018) suggest that the composition of the new PAPs is similar to that of meat meal.
However,new PAPs present:
⇒Lower energy digestibility (8-12% less)
⇒Crude fat (4-25% less)
⇒Protein (8-19% less)
⇒Amino acids and phosphorus (9-19% less) compared to the values found in the CVN tables (2018).
| Therefore, it is advisable to update these values in the ingredient composition tables for animal feed. |
Table 2 shows the composition published by INRAE-CIRAD-AFZ in terms of composition and classification.
- The major essential amino acids are lysine, leucine and valine.
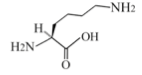
- As for the fat profile, oleic acid holds a big proportion.
![]()
These ingredients are also rich in essential elements such as:

| Maintaining the levels of these elements in accordance with European regulations (Pederiva et al., 2022). |
In regards to protein quality protein and amino acid profile, some studies suggest that depending on the processing method which is employed, heat treatments may favor the rendering of amino acids. This is especially true for aspartic acid. Heat may also reduce the digestibility of protein.
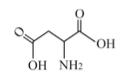
According to a study published by Bellagamba et al. (2015), this reduction in protein digestibility through in vitro studies is maximum at 115ºC for 180 minutes (from 86 to 76 %). Nonetheless, after just 20 minutes of this same treatment, lower protein digestibility was already observed (ranging from 86% to 78.3%).
Table 2. Chemical composition (dry matter) of different types of processed animal proteins
| Sources | INRAE-CIRAD-AFZ (2017-2021)1 | ||||
| Raw Materials | Proteínas procesadas, porcino | Proteínas procesadas aves, 45-60% PB | Proteínas procesadas aves, 60-70% PB | Proteínas procesadas aves, >70% PB | |
| Crude protein | % | 55,1 | 57,5 | 67,5 | 82,2 |
| CF | % | 1,6 | 1,3 | 1,0 | 1,3 |
| NDF | % | 6,4 | 5,1 | 5,1 | 5,1 |
| ADF | % | 2,0 | 3,4 | 3,4 | 3,4 |
| ADL | % | 0,5 | 0,5 | 0,5 | 0,5 |
| Crude Fat | % | 14,3 | 30,8 | 23,2 | 12,1 |
| Starch | % | 0 | 0 | 0 | 0 |
| Ashes | % | 28,2 | 10,9 | 10,7 | 5,5 |
| Calcium | % | 9,12 | 2,09 | 2,32 | 1,53 |
| Phosphorus | % | 4,42 | 1,04 | 1,25 | 0,64 |
| Total Amino Acids | |||||
| Essential | |||||
| Arginine | % | 3,82 | 3,79 | 4,49 | 5,53 |
| Histidine | % | 1,1 | 1,03 | 1,26 | 1,61 |
| Isoleucine | % | 1,61 | 2,31 | 2,69 | 3,25 |
| Leucine | % | 3,34 | 4,01 | 4,78 | 5,91 |
| Lysine | % | 2,79 | 2,64 | 3,27 | 4,21 |
| Methionine | % | 0,71 | 0,81 | 1,17 | 1,0 |
| Fenilalanine | % | 1,89 | 2,26 | 2,69 | 3,33 |
| Threonine | % | 1,84 | 2,3 | 2,71 | 3,33 |
| Valine | % | 2,46 | 3,16 | 3,49 | 3,99 |
| Tryptophan | % | 0,35 | 0,5 | 0,48 | 0,59 |
| Non- essential | |||||
| Alanine | % | 4,24 | 3,2 | 3,77 | 4,6 |
| Aspartate | % | 4,09 | 4,12 | 4,97 | 6,24 |
| Cysteine | % | 0,61 | 1,51 | 0,91 | 2,52 |
| Glutamine | % | 6,53 | 6,6 | 8,04 | 10,2 |
| Glycine | % | 7,02 | 5,12 | 6,03 | 7,39 |
| Proline | % | 4,44 | 4,58 | 4,51 | 4,7 |
| Serine | % | 2,23 | 3,7 | 2,94 | 6,14 |
| Thyroxin | % | 1,24 | 1,5 | 1,85 | 2,36 |
| CE | MJ/kg DM | 18,4 | 25,4 | 24,4 | 23,8 |
| ME rum | MJ/kg DM | – | – | – | – |
| EM pigs(growing) | MJ/kg DM | 11,7 | 16,9 | 17,3 | 19,1 |
| NE pigs (growing) | MJ/kg DM | 8,1 | 12,6 | 12,2 | 12,4 |
| MEn poultry | MJ/kg DM | 12,8 | 19,8 | 18,2 | 16,6 |
| Saturated FAs 3 | |||||
| Lauric, 12:0 | % | 0,01 | 0,02 | 0,02 | 0,008 |
| Miristic, 14 :0 | % | 0,17 | 0,22 | 0,17 | 0,09 |
| Palmitic, 16:0 | % | 2,49 | 4,6 | 3,47 | 1,8 |
| Estearic, 18:0 | % | 1,55 | 1,6 | 1,21 | 0,63 |
| Monounsaturated FAs3 | |||||
| Palmitoleic, 16:1n-7 | % | 0,28 | 1,0 | 0,76 | 0,39 |
| Oleic, 18: 1n-9 | % | 4,34 | 9,33 | 7,03 | 3,66 |
| Polyunsaturated 3 | |||||
| Linoleic, 18:2n-6 | % | 0,95 | 4,12 | 3,1 | 1,61 |
| Linolenic, 18:3n-3 | % | 0,08 | 0,32 | 0,24 | 0,13 |
| Eicosapentaenoic, 20:5n-3 | % | 0 | 0 | 0 | 0 |
| Docosahexanoic, 22:6n-3 | % | 0 | 0 | 0 | 0 |
1 https://feedtables.com/content/table-dry-matter; Values expressed in dry matter
Use in animal feed
At the nutritional level, PAPs are an ingredient of excellent quality:
⇒High content of digestible nutrients (such as amino acids and phosphorus),
⇒ Contain high vitamin content
⇒ No known antinutritional factors, which makes them a candidate to be included in young and adult animals (FEFAC, 2019)
Due to its current low volume, FEFAC (2019) indicates that the poultry feed industry may be the main consumer of these raw materials in the future. Once current barriers in: logistics, organization of facilities (slaughterhouses, feed mills,…), price and public opinion have been overcome.
The inclusion levels of new PAPs in feed are still unknown. However, it is expected that these can be used at a minimum inclusion level of 5%, like old meat meals.
Conclusions
Despite representing local sources of protein, alternative to soybean with good nutritional quality,PAPs still require increased production volumes at lower costs in order for their use to become widespread.
In order for this to occur, logistical barriers involved in the Authorization and Regulation of their production, processing and handling in factories must be overcome.