| The release of phosphorus from phytate occurs through the sequential cleavage of phosphate groups from the inositol ring. It was believed that, regardless of the properties of phytases, the rate of phytate dephosphorylation is limited by the first cleavage of any phosphate group. |

The position of the first phosphate group cleaved depends on the specificity of the phytase. The inhibition of dephosphorylation initiation is not related to the enzyme’s mechanism of action but
rather to insufficient phytase activity or low substrate availability.
» Analysis of transformations in the inositol hexaphosphate(IP6) inositol (I) reaction chain shows that the overall dephosphorylation of IP6 limits the removal of the phosphate group from I(1,2,5,6)P4 (the third reaction from the start of phytic acid phosphate bond hydrolysis).
»Reduced nutrient availability in the presence of phytate is not due to phytase activity, but rather to phytate phosphorus anions (IP6-3) binding to positively charged metal ions, amino acids, and proteins..
| Phytate is a form of phosphorus storage in plant seeds. As a result of phytase activation during seed germination, phytate releases inorganic phosphate (Pi) for use by growing plants. |
| Non-ruminant animals do not efficiently digest phytate present in feed due to the low presence of endogenous enzymes in the gastrointestinal tract (GIT). |
The term phytate includes various substances defined by the presence of the phytic acid anion (PA).
![]()
Enzymes that cleave phytic acid are called myo-inositol hexakisphosphate 3- and 6-phosphohydrolases.
|
|

In solutions with a pH < 1.1, phytic acid has a neutral charge and remains weakly active. An increase in the pH of the medium to 2 leads to the ionization of three phosphate groups; a further increase in pH results in the loss of the remaining protons.
| The phytic phosphorus anion is capable of retaining its negative charge in the gastrointestinal tract and reacting with cations. |
| In the stomach, lysine, histidine, and arginine, as well as proteins and polypeptides containing these amino acids, can form binary protein-IP6 complexes that inhibit protein digestion. |
The binding of phytic acid to positively charged amino acids limits their absorption.
The most common and well-studied are histidine acid phytases, which differ in the carbon atom of the myo-inositol ring from which the first phosphate group is removed.
| »The subsequent removal of phosphate groups results in the release of phosphorus available for plants and animals and the formation of inositol esters with a lower number of soluble phosphates. |
The solubility of isomers depends on the ratio of metal cations and the presence of free amino acids with chelating properties. It is believed that the first three stages of phytic acid dephosphorylation (IP6 IP4) play an important role in creating conditions for the complete hydrolysis of IP.
The negative charge of the phytic acid molecule and its ability to bind to other nutrients decrease as the number of phosphate groups is reduced.
![]()
Alkaline phosphatases and alkaline phytases also participate in the cleavage of isomers.
| Recently, it has been established that when histidine acid phytase is included in the diet (500 units/ kg – 1000 units/kg), it increases the activity of endogenous alkaline phytase in the jejunum, an enzyme that breaks down insoluble divalent metal phytates. |
The action of each type of phytase and its maximum activity depend on the pH of the medium.

| On this basis, phytases are classified as acidic or alkaline. Acid phosphatases exhibit broad substrate specificity toward metal-free phytates, while alkaline phosphatases are specific to metal-bound phytic acid. |
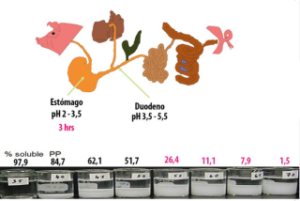
Figure 1: This figure illustrates the gastrointestinal tract of a pig, showing acidity levels in the stomach and duodenum, as well as the retention time of the food bolus in the stomach. The lower portion displays the in vitro solubility of phytate at different pH values.
The solubility of phytate is 97.9% when submerged in a suspension with an acidity of 3.5. This level of acidity is reached when phytate is in the gastric portion of the gastrointestinal tract.
![]() Acidic phytases are highly effective at the stomach level, so it is essential to promote adequate retention time of the food bolus. If acidity increases, phytate precipitation can be observed (a whitish suspension).
Acidic phytases are highly effective at the stomach level, so it is essential to promote adequate retention time of the food bolus. If acidity increases, phytate precipitation can be observed (a whitish suspension).
| In this test, we can observe that the insoluble state of phytate begins to appear at pH 4. In this protonated state, phytate starts binding minerals (Zn, Cu, Co, Mn), forming an insoluble phytate-mineral complex that cannot be absorbed. |
Proteins can also be affected by phytate that has not been properly solubilized, leading to the formation of an insoluble phytate-mineral-protein complex that hinders absorption in pigs.

In vitro studies using the free substrate (sodium phytate), whose availability was not restricted by the cellular structure of feed components, confirmed that IP4 dephosphorylation is the limiting step in the sequential dephosphorylation chain of the original IP6.
| The accumulation of I(1,2,5,6)P4 suggests that its conversion is a “bottleneck reaction” in the sequential dephosphorylation chain from IP6 to IP1. |
![]()
The reduction in the concentration of I(1,2,3,4,6)P5, which retains the phosphate group on the sixth carbon atom in the presence of 6-phytase, suggests that multiple factors may activate alkaline phosphatases and/or inhibit 6-phytase.
Studies on the metabolite group content of IP6 in pig feces confirmed the assumption that the catabolism of IP6, which occurs as a chain of successive dephosphorylation reactions, slows down at the I(1,2,4,6)P4 level.
Factors Affecting the Initiation of Phytic Acid Dephosphorylation
The cell walls of cereal cells are made up of cellulose and hemicelluloses, primarily arabinoxylans, with small amounts of β-glucans and mannans.
Monogastric animals lack enzymes capable of hydrolyzing these cell wall polysaccharides, which means that a portion of the intracellular content of cereal cells remains physically inaccessible to phytases.
| The addition of carbohydrates to the feed facilitates the breakdown of the cell wall and the formation of channels through which water and enzymes can enter the cells, ensuring the digestion of proteins, starch, and phytate. |
» When cellulase was added to the feed, the availability of phosphorus and protein increased by 30.4% and 21.8%, respectively.
»Due to the activity of exogenous proteases, the release of phosphorus increased by 15% and protein by 14.5%.
| The size of the phytase molecules is sufficient for them to penetrate the plant cell walls through the natural pores and catalyze the dephosphorylation of phytate inside the cells. |

Bacterial phytases tend to have smaller molecules compared to fungal enzymes. This suggests that the higher efficacy of bacterial phytases may be due to the size of their molecules, which facilitates access to the substrates.
| An important factor in the activity of phytases is the dissolution of phytate, which allows them to spread beyond plant cells and become available for action. |
In the case of pigs, the food remains in the acidic environment of the stomach for a longer period than in poultry, meaning the amount of soluble phytate should be higher. This explains why pigs utilize phosphorus more efficiently than poultry.
The incomplete cleavage of phytate from natural foods in vivo can be explained by the limited availability of enzymes for the substrate, with the limiting stage being the dephosphorylation of IP4.

⇒The same pattern was observed in other experimental conditions where Escherichia coli, Aspergillus niger, and Buttiauxella sp. phytases were used.
Numerous research articles have demonstrated that phytases have an extra-phosphoric effect, increasing the digestibility of proteins and other nutrients.
| These conclusions were based on the misinterpretation of the results from studies on nutrient utilization, where the physiological digestion process was confused with the processes of amino acid absorption and nitrogen metabolism. |
![]() The extra-phosphoric effect of phytases is understood as an increase in the availability of nutrients other than phosphorus during digestion, meaning it is a manifestation of the effect not associated with the biochemical function of phytases.
The extra-phosphoric effect of phytases is understood as an increase in the availability of nutrients other than phosphorus during digestion, meaning it is a manifestation of the effect not associated with the biochemical function of phytases.
Phytases cannot directly affect protein digestibility; they increase the availability of amino acids already digested by preventing their binding to phosphates, as a result of the reduction in phosphate concentration due to their cleavage.
| Therefore, the concept of the extra-phosphoric effect of phytases is not entirely accurate, as phytase only has one effect. |
Other consequences, although associated with the addition of exogenous phytases to the feed, are a result of their effect on phytate. These are indirect and arise from the reduction of reactive anion concentrations of IP6-4.
Phytate and Piglet Nutrition
Phytate has a significant impact on piglet nutrition, leading to a substantial loss in weight gain if it is not effectively inactivated by phytases.
Piglets have a reduced capacity to produce stomach acidity, as their diet is primarily based on carbohydrates (lactose). As a result, there may be a significant amount of insoluble substrate in the gastrointestinal tract.
| One strategy to help piglets efficiently solubilize phytate is through the application of super doses of phytase. |
» The application of super doses of phytase ensures proper solubilization of this substrate, thus preventing adverse effects on the growth of piglets.
Conclusions
| The digestion of phytates depends on two main factors: the presence of sufficient amounts of phytase and the availability of phytate for enzymatic action. |
1) The first issue can be addressed by adding sufficient amounts of exogenous phytases in appropriate proportions to the feed.
2) The second issue is overcome by increasing the overall digestibility of the feed.
3) In special cases, carbohydrases can be added to the feed, as these enzymes alter cell membranes and facilitate the access of hydrolases to the nutrients in the cytoplasm.
| The cleavage of the first phosphate group by 3 or 6 phytases results in the formation of IP metabolites, including I(1,2,4,5,6)P5 and I(1,2,3,4,5)P5. Compared to IP6, these metabolites bind to nutrients less efficiently, but I(1,2,4,5,6)P5 forms stronger complexes with proteins than I(1,2,3,4,5)P5. |
Additionally, I(1,2,4,5,6)P5 is a precursor of I(1,2,5,6)P4, which inhibits the subsequent stages of IP cleavage. This supports the preference for 6-phytases. The extra-phosphoric effect of phytases, that is, the effect beyond the phosphohydrolase action, is manifested as an increase in the availability of microelements, macroelements, and amino acids.
Phytases do not have proteolytic or amylolytic activity, so the common assumption in popular literature that phytases increase the digestibility of proteins and starch is incorrect.