What are Endotoxins?
Endotoxins are major components of the outer membrane of Gram-negative bacteria.
![]() Endotoxins are composed of a lipid anchor known as lipid A and a repetitive polysaccharide unit, giving them the alternate name of lipopolysaccharides (LPS) (Figure 1).
Endotoxins are composed of a lipid anchor known as lipid A and a repetitive polysaccharide unit, giving them the alternate name of lipopolysaccharides (LPS) (Figure 1).
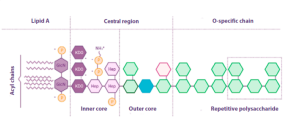
Figura 1. Structure of E. coli endotoxin (adapted from Abate et al., Journal of medical microbiology, 2017).
| Endotoxins elevate the negative charge of the bacterial cell membrane, providing stability to the overall membrane structure. As a result, they create a robust permeability barrier that effectively repels small and hydrophobic molecules. This inherent resistance to various antimicrobial agents makes Gram-negative bacteria naturally resilient. |
While the overall structure of endotoxins is comparable among Gram-negative bacterial species, variations in the composition of polysaccharides (such as specific O chains or O antigens) create a diverse array, distinguishing them between different bacterial strains.
The O antigen plays a crucial role in identifying particular strains of enteric bacteria, such as E. coli (e.g., E. coli O157), with the “O” indicating the presence of the O antigen.
Apart from their role in maintaining bacterial stability and identification, endotoxins play a significant role in human and animal diseases, particularly in relation to immune and inflammatory responses.
What is the biological activity of endotoxins?
The biological activity of endotoxins is linked to the lipid and polysaccharide components of lipopolysaccharides (LPS).
![]() Lipid A is linked to toxicity.
Lipid A is linked to toxicity.
![]() Immunogenicity is associated with polysaccharide components.
Immunogenicity is associated with polysaccharide components.
Lipid A is a potent modulator of the biological response and can stimulate the immune system of mammals.
![]() It binds the endotoxin molecule in the outer membrane of bacterial cells, exerting its biological effects when the endotoxin is released from the cells. This can happen through natural detachment during bacterial growth or when bacteria are lysed due to autolysis, complement attack, phagocytosis, or certain antibiotics.
It binds the endotoxin molecule in the outer membrane of bacterial cells, exerting its biological effects when the endotoxin is released from the cells. This can happen through natural detachment during bacterial growth or when bacteria are lysed due to autolysis, complement attack, phagocytosis, or certain antibiotics.
It is crucial to understand that endotoxins are exceptionally heat-stable and cannot be eliminated through standard autoclaving or heat inactivation methods. Furthermore, unlike bacteria, endotoxins are unaffected by antibiotics and can still induce their toxic effects even in the absence of viable bacteria.
What is the difference between endotoxins and exotoxins?
Endotoxins, by themselves, are not as toxic as other toxins, such as exotoxins.
Exotoxins are proteins that specific strains of both Gram-positive and Gram-negative bacteria produce and secrete. They usually target specific cells where they induce toxic effects, such as altering metabolism and leading to cell death.
For instance, in the case of enterotoxigenic E. coli (ETEC), it generates a heat-stable enterotoxin (STa) that attaches to receptors on enterocytes in the intestine, causing disruption to ion and water transport, leading to severe diarrhea.
In contrast, endotoxins are released upon bacterial lysis and do not have specific target cells.

The immune system “detects” them as signals of Gram-negative bacterial infection, triggering an innate inflammatory immune response.
How do endotoxins stimulate the immune system?
The “toxicity” associated with endotoxins is a result of the host’s immune response. Endotoxins are recognized by the immune system as signals of an infection caused by Gram-negative bacteria, triggering an innate inflammatory immune response.
Even minimal amounts of endotoxins (<1ug) in the bloodstream, which is typically sterile, can induce a significant inflammatory response.
Immune cells, such as macrophages and monocytes, have surface receptors known as Toll-like receptors 4 (TLR4), which recognize endotoxins.

The attachment of the endotoxin to TLR4 triggers a series of cellular signaling events that result in gene expression and the synthesis of proteins that induce an inflammatory response:
![]() Cytokines: Interleukin 6 (IL-6) and TNFα.
Cytokines: Interleukin 6 (IL-6) and TNFα.
![]()
Inflammatory mediators: Platelet-activating factor (PAF) and Interleukin 1 (IL-1).
Endotoxins also activate the Complement system and trigger the blood coagulation system (Figure 2).
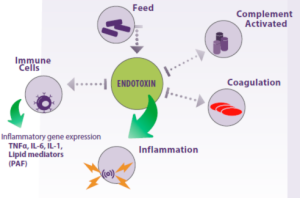
Figure 2. Endotoxins found in animal feed can activate the host’s immune system, leading to an inflammatory response that can potentially harm tissues and organs, ultimately leading to animal death.
This further stimulates inflammatory responses, allowing a rapid activation of the innate immune system to combat any bacteria before the infection progresses too far.
Nevertheless, the strong inflammatory response must be regulated and “switched off” to prevent harm to the host.
![]() This balance is achieved by producing anti-inflammatory mediators, such as interleukin 10 receptor antagonists and interleukin 1 receptor antagonists, along with proteins that bind to endotoxins, like lipopolysaccharide-binding protein (LBP).
This balance is achieved by producing anti-inflammatory mediators, such as interleukin 10 receptor antagonists and interleukin 1 receptor antagonists, along with proteins that bind to endotoxins, like lipopolysaccharide-binding protein (LBP).
Additionally, immune cells such as macrophages and neutrophils can help eliminate endotoxins by producing enzymes that degrade the lipid A portion of the endotoxin molecule.
The liver also plays an important role in this process.
| In the event of a substantial influx of endotoxins into the bloodstream or their delayed clearance, the inflammatory response may intensify, causing a “cytokine storm” that overwhelms the regulatory capacity of anti-inflammatory mechanisms. This can result in damage to the host’s tissues and organs. |
This condition is known as sepsis and can lead to a high mortality rate.
As a result, the presence of endotoxins in the bloodstream can be fatal, and measures must be taken to minimize their entry.
How are endotoxins detected?
Throughout evolution, the response to endotoxins has been preserved, bestowing our ancestors with a survival advantage in a world full of Gram-negative pathogens.
In fact, a response to endotoxins has been observed in ancient marine creatures like the horseshoe crab (Limulus polyphemus), which has been on Earth for millions of years (Levin and Bang, 1968).
 The identification of an immune clotting response to endotoxins in horseshoe crabs paved the way for the creation of a remarkably sensitive test to detect bacterial endotoxins. This test utilizes the blood cells (amebocytes) of these organisms and is known as the Limulus Amebocyte Lysate (LAL) test (Figure 3).
The identification of an immune clotting response to endotoxins in horseshoe crabs paved the way for the creation of a remarkably sensitive test to detect bacterial endotoxins. This test utilizes the blood cells (amebocytes) of these organisms and is known as the Limulus Amebocyte Lysate (LAL) test (Figure 3).
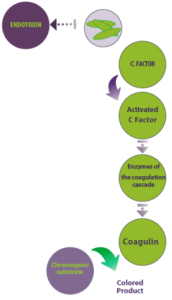
Figure 3 illustrates the principle of the LAL test, which is utilized to detect bacterial endotoxins. In this process, endotoxins from Gram-negative bacteria bind and activate Factor C in Limulus amebocytes, initiating a series of enzyme reactions that culminate in the formation of a blood clot (coagulin). The addition of a chromogenic substrate enables the reaction to produce a colored product.
The LAL test is a standard procedure in the pharmaceutical industry, ensuring that all medications and medical devices, including pacemakers, catheters, and other invasive instruments, are free from endotoxin contamination. This stringent testing is essential as the inadvertent introduction of endotoxins into the human body could trigger potentially fatal immune reactions (sepsis) (Abate et al., 2020).
 Molendotech Limited researchers have created a modified LAL test capable of analyzing environmental samples to detect endotoxin levels, including bacterial or fecal contamination in water, food, and animal feed. Molendotech Limited researchers have created a modified LAL test capable of analyzing environmental samples to detect endotoxin levels, including bacterial or fecal contamination in water, food, and animal feed. |
How can endotoxins enter the bloodstream?
The intestine is a massive reservoir of Gram-negative bacteria and consequently contains large amounts of endotoxins that can enter the bloodstream through intestinal absorption.
The body has developed compartmentalization as a protective mechanism to prevent excessive amounts of endotoxins from entering the bloodstream.
 Nevertheless, intestinal injuries, a diet rich in fats, pharmacological treatments, infections, or immaturity (e.g., neonates) can facilitate the translocation of endotoxins through the membrane and into the systemic circulation.
Nevertheless, intestinal injuries, a diet rich in fats, pharmacological treatments, infections, or immaturity (e.g., neonates) can facilitate the translocation of endotoxins through the membrane and into the systemic circulation.
In animals, impairment of intestinal barrier function is also a concern, and stress in pigs or subacute ruminal acidosis (SARA) in cattle can facilitate the entry of endotoxins into the bloodstream, resulting in pathological consequences.
![]()
Low doses of endotoxins have been shown to induce SARA symptoms, including inflammation, decreased ruminal pH, and alterations in the microbiota (Jing et al., 2014). Therefore, inhalation or ingestion of endotoxins can trigger severe disease in young animals.
Gram-negative bacteria, particularly E. coli, are extensively utilized in recombinant technology for producing feed additives such as amino acids and vitamins.
If endotoxins from contaminated bacteria enter the additives, it may pose a risk to both animals and workers handling the additives, as well as consumers.
It has been documented, for example, that workers exposed to inhaled endotoxins from poultry house dust or other sources present clinical symptoms, including decreased lung function (Health Council of the Netherlands, 2010).
 While farm animals are consistently exposed to endotoxins from their environment, including feed, diets with elevated endotoxin levels carry a risk. Even small doses that manage to cross the intestinal barrier can lead to serious diseases (Mani et al., 2013). While farm animals are consistently exposed to endotoxins from their environment, including feed, diets with elevated endotoxin levels carry a risk. Even small doses that manage to cross the intestinal barrier can lead to serious diseases (Mani et al., 2013). |
![]()
Hence, it is prudent to restrict the inclusion of feed ingredients containing endotoxins in animals with compromised gastrointestinal barrier function (Wallace et al., 2016).
Animals are also expected to react to inhaled endotoxins present in feed and poultry dust similarly to human workers. However, there are few studies on animal exposure to inhaled endotoxins, and exposure limits are uncertain.
Can mycotoxins influence the response to endotoxins?
Mycotoxins are toxins produced by fungi (molds) that grow on crops or feed ingredients during storage. They can cause a range of adverse health effects and pose a serious threat to the health of humans and livestock.
![]() Mycotoxins commonly found in feed include aflatoxins, zearalenone, T2 toxin, deoxynivalenol, and ochratoxin A.
Mycotoxins commonly found in feed include aflatoxins, zearalenone, T2 toxin, deoxynivalenol, and ochratoxin A.
Feeds contaminated with mycotoxins and endotoxins lead to the simultaneous presence of both substances in the gastrointestinal tract of production animals. Studies have revealed that this combined presence exerts synergistic adverse effects on animal health, causing subsequent economic impacts.
As mentioned earlier, an intact intestinal barrier is crucial to reducing the incorporation of endotoxins into the bloodstream and preventing their inflammatory consequences.
Furthermore, a well-functioning immune system and liver play a crucial role in regulating the level of endotoxins in the bloodstream.
![]() Mycotoxins damage the intestinal epithelium, allowing more intestinal contents, including endotoxins, to enter the bloodstream.
Mycotoxins damage the intestinal epithelium, allowing more intestinal contents, including endotoxins, to enter the bloodstream.
Additionally, mycotoxins impact the functioning of immune cells and cause liver damage, resulting in heightened inflammatory responses and reduced elimination mechanisms for endotoxins (Figure 4).
Thus, mycotoxins present in animal feeds act synergistically with endotoxins, causing severe consequences for the health of animals that consume contaminated feeds. Young animals, especially during the weaning phase, are particularly vulnerable (Wallace et al., 2016).
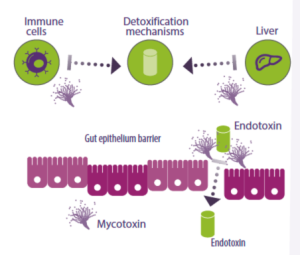
Figure 4. Mycotoxins affect immune cells and the liver, hindering the production of endotoxin detoxification enzymes and other anti-inflammatory molecules. They can also alter the intestinal epithelial barrier, which typically prevents the entry of endotoxins into the bloodstream.
How can we mitigate the presence of endotoxins and mycotoxins in animal feed?
In order to mitigate the effects of endotoxins and mycotoxins in feeds, it is essential to treat them with substances that can bind and remove these molecules (Boyacioglu, 2019), and it is crucial to validate their efficacy in eliminating endotoxins.
Additionally, feed samples can undergo testing to assess the presence of Gram-negative bacteria and endotoxins.
 Tests for various mycotoxins do exist, but they tend to be more complex. However, there are promising developments in the pipeline, and soon, general screening for fungal contamination should become more accessible with the availability of new tests.
Tests for various mycotoxins do exist, but they tend to be more complex. However, there are promising developments in the pipeline, and soon, general screening for fungal contamination should become more accessible with the availability of new tests.
Source: This article was originally published as a content in spanish on nutriNews 2023