Mycotoxins in grains and concentrates for ruminants
INTRODUCTION
Mycotoxin-producing fungi thrive on animal and human feedstuffs, utilizing them as their growth medium. These fungi generate mycotoxins, which are secondary metabolites characterized by their low molecular weight and high toxicity (Zain, 2011). Grains and plant-based concentrates, in particular, serve as optimal substrates for their proliferation (Yiannikouris and Jouany, 2002).
Fungal colonization of grains can occur either before or after harvesting.
| Mycotoxins have the potential to be generated at both the exponential growth and stationary phases of fungal development, posing a substantial threat to animal and human health (Bullerman and Draughon, 1994). Human exposure to mycotoxins can result from the consumption of contaminated plant-based foods or by consuming animal-derived products from animals that have themselves ingested tainted feed (WHO, 2018). |
EFFECTS IN RUMINANTS
 In general, animals are exposed to mycotoxin intoxication based on the type of feed they consume, whether it be grains or concentrates. Ruminants are more resistant to the toxic effects of mycotoxins compared to monogastrics, and this increased resistance is attributed to the ruminal microbiota, which degrades and inactivates these metabolites(Fink-Gremmels, 2008). Nevertheless, numerous toxic effects associated with the consumption of mycotoxin-contaminated food are still described.
In general, animals are exposed to mycotoxin intoxication based on the type of feed they consume, whether it be grains or concentrates. Ruminants are more resistant to the toxic effects of mycotoxins compared to monogastrics, and this increased resistance is attributed to the ruminal microbiota, which degrades and inactivates these metabolites(Fink-Gremmels, 2008). Nevertheless, numerous toxic effects associated with the consumption of mycotoxin-contaminated food are still described.
|
The main genera responsible for mycotoxin contamination in grains meant for ruminants are Fusarium, Aspergillus, and Penicillium (Bonifaz, 2012). |
![]() Fusarium fungi are widespread and often contaminate crops during their development, especially when conditions of humidity and temperature are favorable.
Fusarium fungi are widespread and often contaminate crops during their development, especially when conditions of humidity and temperature are favorable.
![]() Several species within this genus produce mycotoxins that can cause intoxications in both ruminants and humans, as well as other animals (Zinedine et al., 2007). The main toxins produced by this genus of fungi are fumonisins, trichothecenes, and zearalenone.
Several species within this genus produce mycotoxins that can cause intoxications in both ruminants and humans, as well as other animals (Zinedine et al., 2007). The main toxins produced by this genus of fungi are fumonisins, trichothecenes, and zearalenone.
![]() Fumonisins are of significant concern as they interfere with sphingolipid metabolism (Marasas, 1995). The most important fumonisin, fumonisin B1, has been linked to esophageal cancer in humans (EFSA, 2005). The International Agency for Research on Cancer (IARC) considers it a possible human carcinogen (Group 2B).
Fumonisins are of significant concern as they interfere with sphingolipid metabolism (Marasas, 1995). The most important fumonisin, fumonisin B1, has been linked to esophageal cancer in humans (EFSA, 2005). The International Agency for Research on Cancer (IARC) considers it a possible human carcinogen (Group 2B).
 Among animals, fumonisins have been subject to more comprehensive investigation in swine and equines. Ruminants, notably beef cattle, seem to exhibit greater resistance to their adverse effects. Nevertheless, it is well-documented that fumonisins are minimally metabolized in the rumen, leading to hepatotoxic and nephrotoxic consequences, as well as detrimental impacts on feed intake and production in dairy cows (Smith, 2012).
Among animals, fumonisins have been subject to more comprehensive investigation in swine and equines. Ruminants, notably beef cattle, seem to exhibit greater resistance to their adverse effects. Nevertheless, it is well-documented that fumonisins are minimally metabolized in the rumen, leading to hepatotoxic and nephrotoxic consequences, as well as detrimental impacts on feed intake and production in dairy cows (Smith, 2012).
![]() Trichothecenes like deoxynivalenol (DON) and T-2 toxin undergo metabolic transformation in the rumen, resulting in the production of considerably less harmful metabolites. As a result, they are unlikely to induce substantial disruptions in ruminant animals, although it’s worth noting that the available information on this subject is limited, as highlighted by (Eriksen and Pettersson in 2004).
Trichothecenes like deoxynivalenol (DON) and T-2 toxin undergo metabolic transformation in the rumen, resulting in the production of considerably less harmful metabolites. As a result, they are unlikely to induce substantial disruptions in ruminant animals, although it’s worth noting that the available information on this subject is limited, as highlighted by (Eriksen and Pettersson in 2004).

Zearalenone has a three-dimensional molecular configuration similar to estradiol, enabling it to occupy estradiol receptors, stimulating them and acting as an endocrine disruptor in males and females of various animal species (D’Mello et al., 1999; Haschek et al., 2002).
|
In males, a feminization syndrome is observed, with reduced testosterone levels, testicular weight, spermatogenesis, and libido (Zinedine et al., 2007). |
![]() Meanwhile, fungal species belonging to the Aspergillus and Penicillium genera primarily proliferate during storage. Aspergillus spp. is notably recognized for its capacity to generate potent toxins such as aflatoxins and ochratoxins, as outlined by (Navale et al., 2021).
Meanwhile, fungal species belonging to the Aspergillus and Penicillium genera primarily proliferate during storage. Aspergillus spp. is notably recognized for its capacity to generate potent toxins such as aflatoxins and ochratoxins, as outlined by (Navale et al., 2021).
Aflatoxins exhibit hepatotoxic, immunosuppressive, mutagenic, teratogenic, and carcinogenic properties across various species, including humans, according to (Zain ,2011). Among these toxins, aflatoxin B1 stands out as the most potent naturally occurring carcinogen known, as noted by (Coppock et al., 2018).
![]()
 In ruminants, the effects can range from acute to chronic, contingent on the dose and duration of exposure. Chronic intoxication is particularly concerning for public health in dairy cows, given that both aflatoxin B1 and its milk-derived metabolite, M1, are carcinogenic in humans (IARC, 1993; IARC, 2002) and also contribute to immunosuppression in nursing calves.
In ruminants, the effects can range from acute to chronic, contingent on the dose and duration of exposure. Chronic intoxication is particularly concerning for public health in dairy cows, given that both aflatoxin B1 and its milk-derived metabolite, M1, are carcinogenic in humans (IARC, 1993; IARC, 2002) and also contribute to immunosuppression in nursing calves.
![]() Ochratoxins, produced by several Penicillium species, possess nephrotoxic and immunosuppressive properties, as detailed by Perrone and Susca in 2017. Ochratoxin A, while highly potent, rarely exerts negative effects on ruminants, as it is converted into less active compounds within the rumen by protozoa, as reported by (Mobashar et al.,2010).
Ochratoxins, produced by several Penicillium species, possess nephrotoxic and immunosuppressive properties, as detailed by Perrone and Susca in 2017. Ochratoxin A, while highly potent, rarely exerts negative effects on ruminants, as it is converted into less active compounds within the rumen by protozoa, as reported by (Mobashar et al.,2010).
| Table 1 provides a summary of the primary effects observed as a result of mycotoxin consumption in ruminants, based on a review by Gallo and co-authors in 2015. It underscores the relatively limited and inconclusive knowledge available on the topic. |
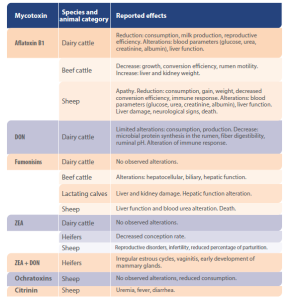
Table 1. Key effects of mycotoxin exposure in ruminants observed in experimental or field studies (summarized from Gallo et al., 2015)
|
Beyond specific actions on tissues or organs, mycotoxins rarely lead to acute intoxications, and the symptoms in ruminants are often quite nonspecific. Decreased production or consumption without apparent cause, food refusal, increased susceptibility to diseases, reproductive failures, or abortions may have their origin in the presence of mycotoxins in the feed, although many times this presence may go unnoticed if the diagnosis is not performed correctly. |
INITIATION OF CONTAMINATION AND ENVIRONMENTAL CONDITIONS FOR DEVELOPMENT
 The growth of fungi and subsequent mycotoxin production is not constant but contingent on environmental factors, including humidity and temperature. Generally, elevated temperature and humidity conditions promote both fungal growth and mycotoxin production.
The growth of fungi and subsequent mycotoxin production is not constant but contingent on environmental factors, including humidity and temperature. Generally, elevated temperature and humidity conditions promote both fungal growth and mycotoxin production.
|
Fungi can thrive in food without necessarily producing mycotoxins; however, they synthesize these toxins in response to specific stressors. Extreme weather conditions like drought or excessive humidity, the presence of damaged grains, or inadequate crop and storage management can act as stress factors triggering mycotoxin production, as indicated by (Whitlow y Hagler, 2005). |
 In this context, events associated with climate change appear to be altering patterns of mycotoxicosis outbreaks, causing them to manifest in regions where they were previously uncommon, as observed by (Tolosa et al., 2021).
In this context, events associated with climate change appear to be altering patterns of mycotoxicosis outbreaks, causing them to manifest in regions where they were previously uncommon, as observed by (Tolosa et al., 2021).
The contamination of grains with fungi (and consequently mycotoxin levels) is not uniform, with some areas within batches, silos, or storage facilities showing higher contamination levels than others, forming “accumulation hotspots” according to the Food Safety Authority of Ireland in 2009. Even grains within the same batch can exhibit variation, as highlighted by(Tittlemier et al., 2022).
|
It is essential to consider these variations when sampling grains for mycotoxin detection. Specific protocols must be followed for the type of material and storage, involving the extraction of material from various areas, considering different depths and heights. |
 In the case of humid sorghum grains stored in bags, García and Santos et al. (2020)reported a beneficial effect of storage time. If storage occurs under suitable conditions, the sustained low pH over time reduces the concentration of condensed tannins (which can be potential protectors against fungal contamination in the grain but hinder ruminal fermentation) and reduces fungal contamination.
In the case of humid sorghum grains stored in bags, García and Santos et al. (2020)reported a beneficial effect of storage time. If storage occurs under suitable conditions, the sustained low pH over time reduces the concentration of condensed tannins (which can be potential protectors against fungal contamination in the grain but hinder ruminal fermentation) and reduces fungal contamination.

According to this study, storing grains in bags for 180 days would be optimal for enhancing ruminal fermentation of hard-to-digest grains and reducing contamination. As reported by García and Santos et al. (2022), in sorghum grain silos, the relative abundance of Fusarium decreased after 30 days of storage, and in grains with high tannin content, Aspergillus spp. decreased.
These findings open up a new perspective on the potential advantages of using grains with high tannin content to produce silage, at least in environments with a high risk of fungal contamination.
| A notable attribute of mycotoxins is their resilience against food processing methods. They endure drying, grinding, and exhibit exceptional thermal stability, rendering their eradication through cooking a formidable challenge (Kabak, 2009). These inherent properties significantly complicate their management, reinforcing the notion among nutritionists that when it comes to mycotoxin control, “prevention is far more effective than the cure.” |
![]() Within this framework, the following guidance is provided concerning food: the identification and measurement of mycotoxin-producing fungi and the assessment of mycotoxin levels. Armed with this data, steps can be implemented to reduce the likelihood of contamination in food meant for both human and animal consumption.
Within this framework, the following guidance is provided concerning food: the identification and measurement of mycotoxin-producing fungi and the assessment of mycotoxin levels. Armed with this data, steps can be implemented to reduce the likelihood of contamination in food meant for both human and animal consumption. 
IDENTIFICATION & QUANTIFICATION
Historically, the identification and quantification of toxigenic fungi contaminating food have been carried out through isolation and morphological identification based on their phenotypic characteristics. In these methods, the developed colonies of isolated food cultures are counted and transferred to specific media for identification under a microscope based on their micro and macro-morphological characteristics according to conventional identification keys for the main fungal genera.  |
| These techniques demand extensive labor, a substantial level of expertise, and time-consuming efforts. Currently, molecular methods utilizing PCR for identification and quantification have emerged as alternatives, addressing the issues mentioned previously.. |
 These methods allow the identification of isolates at the species level by amplifying and sequencing different genes (Ward et al., 2002; O’Donnell et al., 2004) or by amplification with specific primers (Nicolaisen et al., 2009; Scauflaire et al., 2012).
These methods allow the identification of isolates at the species level by amplifying and sequencing different genes (Ward et al., 2002; O’Donnell et al., 2004) or by amplification with specific primers (Nicolaisen et al., 2009; Scauflaire et al., 2012).
To determine and quantify mycotoxin concentrations in food, various immunoassay and chromatographic methods can be employed (Diaz and Smith, 2005). Chromatographic techniques such as thin-layer chromatography (TLC), high-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), and liquid chromatography-mass spectrometry (LC-MS).
The latter method, LC-MS, is widely used due to its potential for evaluating large quantities of samples and simultaneously detecting various mycotoxins (Krska et al., 2008). Another methodology currently in use is a combined and integrated rapid immunochromatographic technique.
This method combines antibodies on a single membrane strip, allowing the detection of various analytes in just minutes. It requires a portable lateral flow chromatography device that enables the determination of a wide range of mycotoxin concentrations on-site.
CONTROL + PREVENTION

To control and prevent the contamination of food by toxigenic fungi and mycotoxins, it is essential to initiate management practices in the field. This encompasses considerations such as the choice of crops, crop varieties, weed control, irrigation, and crop rotation (Edwards, 2004). Given that climatic conditions are beyond control and significantly impact fungal and mycotoxin development, field-level measures may not always be entirely effective, necessitating intervention during harvesting and storage.
During harvest, grain damage should be avoided, as it predisposes to fungal contamination and mycotoxins. In storage, it may be possible to control humidity and temperature to reduce the risk of contamination (Shapira & Paster, 2004). Acidic environments and low water activity are effective ways to control and inhibit bacterial growth. However, fungi can grow under a broader range of physicochemical conditions than most bacteria.
| In food products, fungi proliferate under pH ranges of 2 to 9, with water activities ranging from 0.61 to 0.99 (Snyder et al., 2019). In storage, substances inhibiting fungal growth can be used, but these do not act on mycotoxin content if it already exists. |
| In cases of mycotoxin contamination in food, one method employed for control involves the application of sequestering agents. These agents are typically high-molecular-weight polymers, either inorganic or organic, that serve to diminish the absorption of mycotoxins within the digestive tract, consequently reducing their toxicity within the animal’s system.
To accomplish this, sequestrants create irreversible complexes with these toxins in the intestinal lumen, and these complexes are subsequently excreted in the feces (Devegowda and Murthy, 2005).
|
References:
Bonifaz, A., 2012. Micología clínica básica. 4 edición. Mcgraw Hill.
Bullerman, I., Draughon, F., 1994. Fusarium moniliforme and fumonisin symposium-introduction. Journal of Food Protection 57(6): 513
Coppock R.W., Christian R.G., Jacobsen B.J. 2018 Aflatoxins. En R. Gupta (Ed). Veterinary Toxicology. 3°Ed. (pp 983-994). Academic Press. https://doi.org/10.1016/B978-0-12-811410-0.00069-6
D’Mello, J.P.F., Placinta, C.M., McDonald, A.M.C., 1999. Fusarium mycotoxins: a review of global implications for animal health, welfare and productivity. Animal Feed Science and Technology 80, 183-205. https://doi.org/10.1016/S0377-8401(99)00059-0
Devegowda, G., Murthy, T., 2005. Mycotoxins: effect on poultry performance and health. En: The Mycotoxin Blue Book. Ed. Duarte Díaz, Nottingham University Press, 25-56.
Diaz, D.E., Smith, T.K., 2005. Mycotoxin sequestering agents: practical tools for the neutralisation of mycotoxins. The mycotoxin blue book. Ed. Duarte Díaz, Nottingham University Press, 323-339.
Edwards, S. G., 2004. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicology Letters, 153, 29–35. https://doi.org/10.1016/j.toxlet.2004.04.022
EFSA (European Food Safety Authority), 2005. Draft Opinion of the Scientific Committee on a harmonized approach for risk assessment of compounds which are both genotoxic and carcinogenic. http://www.efsa.eu.int/science/sc_commitee/sc_consultations/882_en.html
Eriksen, G.S., Pettersson, H., 2004. Toxicological Evaluation of Trichothecenes in Animal Feed. Animal Feed Science and Technology 114, 205-239. https://doi.org/10.1016/j.anifeedsci.2003.08.008
Fink-Gremmels, J., 2008. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 176, 84-92. https://doi.org/10.1016/j.tvjl.2007.12.034
Food Safety authority of Ireland, 2009. Mycotoxins in Food. Toxicology factsheet series, Issue 1.https://www.fsai.ie/getmedia/4142c320-cc5a-41d7-bb9d-09517f182501/mycotoxins.pdf?ext=.pdf
Gallo A., Giuberti G., Frisvad C., Bertuzzi T., Nielsen K.F. 2015. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 7, 3057-3111. https://doi.org/10.3390/toxins7083057
García y Santos, C., Bettucci, L., Brambillasca, S., Cajarville, C., 2020. Storage time and condensed tannin content of high-moisture sorghum grains: Effects on in vitro fermentation and mold populations. Anim. Nutr. 6:92-97. https://doi.org/10.1016/j.aninu.2019.08.002
García y Santos C., Cajarville C., Suárez G., Bettucci L. 2022. How do Time, Tannin, and Moisture Content Influence Toxicogenic Fungal Populations during the Storage of Sorghum Grains? Journal of Food Protection, 85, 5, 778-785. https://doi.org/10.4315/JFP-21-239
Haschek W.M., Voss K., BeasleyV. 2002. Selected Mycotoxins Affecting Animal and Human Health W.M. Haschek, C.G. Rousseaux, M.A. Wallig (eds.) Handbook of Toxicologic Pathology (Second Ed.), Academic Press, pp: 645-699, https://doi.org/10.1016/B978-012330215-1/50026-0
Hoerr, F.J., 2003. Mycotoxicoses. En: Diseases of Poultry. Saif, Y.M. ed. Iowa State University Press, Ames, Iowa, USA. p 1103-1132.
IARC, 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 56, Some Naturally Occurring Substances: Food Items and Constituents, Hetero-cyclic Amines and Mycotoxins. IARC Press, Lyon, Francia. pp. 245-297.
IARC, 2002. Aflatoxins. IARC monographs on the evaluation of carcinogenic risk to humans: Some traditional herbal medicines, some mycotoxins, naphthalene and sty-rene. World Health Organization, 82:171-249.
Kabak, B., 2009. The fate of mycotoxins during thermal food processing. Journal of the Science of Food and Agriculture, 89, 4, 549-554. https://doi.org/10.1002/jsfa.3491
Krska, R., Schubert-Ullrich, P., Molinelli, A., Sulyok, M., MacDonald, S., Crews, C., 2008. Mycotoxin analysis: An update. Food additives and contaminants, 25, 2, 152-163. https://doi.org/10.1080/02652030701765723
Liu J., Applegate T. 2020. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 12, 377. https://doi.org/10.3390/toxins12060377
Marasas, W.F.O. 1995. Fumonisins: Their implications for human and animal health. Natural Toxins 3, 4, 193-198. https://doi.org/10.1002/nt.2620030405
Mobashar M, Hummel J, Blank R, Südekum KH. 2010. Ochratoxin A in ruminants−A review on its degradation by gut microbes and effects on animals. Toxins (Basel) 2, 809-39. https://doi.org/10.3390/toxins2040809
Navale V, Vamkudoth KR, Ajmera S, Dhuri V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection, and toxicity. Toxicol Rep. 2021 May 2;8:1008-1030. https://doi.org/10.1016/j.toxrep.2021.04.013.
Nicolaisen, M., Supronienė, S., Nielsen, L. K., Lazzaro, I., Spliid, N. H., Justesen, A. F., 2009. Real-time PCR for quantification of eleven individual Fusarium species in cereals. Journal of Microbiological Methods, 76, 3, 234-240. https://doi.org/10.1016/j.mimet.2008.10.016
O’Donnell, K., Ward, T.J., Geiser, D.M., Corby Kistler, H., Aoki, T., 2004. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genetics and Biology 41, 600-623. https://doi.org/10.1016/j.fgb.2004.03.003
Perrone G., Susca A. 2017. Penicillium Species and Their Associated Mycotoxins. Methods Mol Biol. 1542: 107-119. https://doi.org/10.1007/978-1-4939-6707-0_5.
Pestka, J.J., 2007. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Animal Feed Science and Technology 137, 283-298. https://doi.org/10.1016/j.anifeedsci.2007.06.006
Scauflaire, J., Godet, M., Gourgue, M., Liénard, C., Munaut, F., 2012. A multiplex real-time PCR method using hybridization probes for the detection and the quantification of Fusarium proliferatum, F. subglutinans, F. temperatum, and F. verticillioides. Fungal biology, 116, 10, 1073-1080. https://doi.org/10.1016/j.funbio.2012.07.011
Shapira, R., Paster, N., 2004. Control of mycotoxins in storage and techniques for their decontamination. In M. Magan, N. Olsen (Ed.), Mycotoxins in Food. Detecction and Control. (pp. 190–223). Elsevier. https://doi.org/10.1533/9781855739086.2.190
Smith, G. 2012. Fumonisins. En R. Gupta (Ed). Veterinary Toxicology. 2°Ed. (pp. 1205-1219). Elsiever. https://doi.org/10.1016/B978-0-12-385926-6.00104-6
Snyder, A.B., Churey, J. J., Worobo, R.W., 2019. Association of fungal genera from spoiled processed foods with physicochemical food properties and processing conditions Food Microbiology. 83: 211-218. https://doi.org/10.1016/j.fm.2019.05.012
Tittlemier S.A., Cramer B., Dall’Asta C., DeRosa M.C., Lattanzio V.M.T., Malone R., Maragos C., Stranska M., Sumarah M.W. 2022. World Mycotoxin Journal 15, 1-25. https://doi.org/10.3920/WMJ2021.2752
Tolosa, J., Rodríguez-Carrasco, Y., Ruiz, M., & Vila-Donat, P. (2021). Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food and Chemical Toxicology, 158, 112661. https://doi.org/10.1016/j.fct.2021.112661
Ward, T.J., Bielawski, J.P., Kistler, H.C., Sullivan, E., O’Donnell, K., 2002. Ancestral polymorphism and adaptive evolution in the trichothecene mycotoxin gene cluster of phytopathogenic Fusarium. Proceedings of the National Academy of Sciences of the United States of America 99, 9278–9283. https://doi.org/10.1073/pnas.142307199
Whitlow, L.W., Hagler, W.M., 2005. Mycotoxins in dairy cattle: Occurrence, toxicity, prevention and treatment. Proc. Southwest Nutr. Conf. 124-138.
World Health Organization (WHO), 2018. Micotoxinas. https://www.who.int/es/news-room/fact-sheets/detail/mycotoxins
Yiannikouris, A., Jouany, J.P., 2002. Mycotoxins in feeds and their fate in animals: a review. Animal Research 51, 81-99.
Zain, M., 2011. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 15:129-144. https://doi.org/10.1016/j.jscs.2010.06.006.
Zinedine A, Soriano JM, Moltó JC, Mañes J., 2007. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem Toxicol. 45, 1-18.