 15 Mar 2022
15 Mar 2022
Tryptophan: impact on the gut microbiota of animals. Gut microbiota and microbial metabolites are important for maintaining healthy intestines. It influences the extent and quality of the immune system’s response; and in turn the immune system is involved in regulating the location and composition of gut microbiota.
 Recent studies highlight the profound effects of diet and nutrients on the location and composition of the gut microbiota. In addition to underscoring its connection with immune pathways (Thorburn et al., 2014).
Recent studies highlight the profound effects of diet and nutrients on the location and composition of the gut microbiota. In addition to underscoring its connection with immune pathways (Thorburn et al., 2014). 
L-tryptophan (Trp) and its endogenous metabolites are involved in intestinal immune homeostasis. Manipulation of intestinal microbial composition can modulate plasma concentrations of Trp and its metabolites (Clarke et al. , 2014). The microbiome, which consists of microorganisms and their collective genomes, modulates the host’s metabolic phenotype and influences its immune system (Gordon, 2012). Interactions between the gut microbiota and the host’s immune system begin at birth:
![]()
[register] Later in life, the gut microbiota also influences immune cell recruitment and initiates inflammation. Crosstalk between gut microbiota and enterocytes shapes the gut environment and profoundly influences gut immune homeostasis (Hold, 2016), which lasts a lifetime. Alterations in the gut microbiota, along with increased intestinal permeability (leaky gut), are widely recognized as relevant to the pathogenesis of several diseases (Anderson et al., 2016). The connection between microorganisms and the host’s immune system is mediated by a number of molecules (Anders et al. , 2013) and signaling processes, which can affect the gut, liver, brain, and other organs.
On the other hand, the intestinal immune system plays a crucial role in exposing bacteria to host tissues, alleviating potential pathological outcomes and determining the stratification of gut bacteria on the luminal side of the epithelial barrier. (Blumberg y Powrie, 2012). 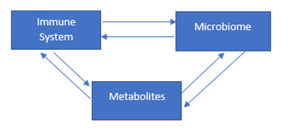 Tryptophan’s impact on gut health
Tryptophan’s impact on gut health
In addition to serving as a substrate for protein synthesis, Tryptophan is metabolized primarily through two metabolic pathways:
Approximately 95% of ingested Trp is degraded to kynurenine, kynurenic acid (KA), quinolinic acid, picolinic acid, and nicotinamide adenine dinucleotide (NAD). While 1-2% of ingested Trp is converted into serotonin (5-HT) and melatonin. The gut microbiota can directly use Trp, which partially limits the amino acid’s availability for the host. Reports have shown that the bacterial community can influence Tryptophan metabolism and the serotonergic system. Total circulating Trp levels increase in  germ-free (LG) mice lacking gut microbiota (El Aidy et al., 2012; Mardinoglu et al., 2015). Circulating serotonin concentrations also decrease (Clarke et al., 2013). In turn, host Trp depletion can reduce microbial proliferation and affect intestinal immunity (Hashimoto et al., 2012).
germ-free (LG) mice lacking gut microbiota (El Aidy et al., 2012; Mardinoglu et al., 2015). Circulating serotonin concentrations also decrease (Clarke et al., 2013). In turn, host Trp depletion can reduce microbial proliferation and affect intestinal immunity (Hashimoto et al., 2012).
Kynurenines have antimicrobial activities, which can directly impact the proliferation of the intestinal microbiota (Niño-Castro et al., 2014).

![]()
Bacterial species such as E. coli, Proteus vulgaris, Paracolobactrum coliform, Achromobacter liquefaciens and Bacteroides spp are capable of producing indole (Keszthelyi et al. , 2009).
Recently, indole has been recognized as a signaling molecule that can regulate bacterial motility, biofilm formation, antibiotic resistance, persistent cell formation, and virulence (Li and Young, 2013). In the pig gut on a diet low in non-starchy polysaccharides, the maximum concentration of indole (~0.12 mM) was found in the distal part of the cecum, where most gut bacteria had settled, while the amount of indole in the hind gut was lower in animals fed a diet high in non-starchy polysaccharides (Knarreborg et al. , 2002).
![]()
Indole exposure can strengthen the mucosal barrier and mucin production by inducing the expression of associated genes, which increases resistance to pathogen invasion. For inflammatory cells, indole exposure can suppress NF-κB activation and pro-inflammatory chemokine production and, at the same time, increase the production of anti-inflammatory cytokines, thereby improving inflammation and damage.
 Eschatol is another metabolite derived from the gut microbiota of Tryptophan (Jensen et al., 1995). It can influence the growth and reproduction of certain gut bacteria and has bacteriostatic effects on Gram-negative enterobacteria.
Eschatol is another metabolite derived from the gut microbiota of Tryptophan (Jensen et al., 1995). It can influence the growth and reproduction of certain gut bacteria and has bacteriostatic effects on Gram-negative enterobacteria.
![]()
Eschatol production is associated with both health and disease states: In cattle, intraruminal and intravenous administration of eschatol induces clinical features and lung lesions similar to Tryptophan-induced disease.
Tryptamine is a neurotransmitter that acts on the enteric nervous system to modulate intestinal homeostasis (Wlodarska et al., 2015) and can induce the secretion of ions by intestinal epithelial cells.
Conclusions
Our knowledge about the interactions between Trp metabolism, gut microbiota and host immunity has expanded considerably in recent years. Growing evidence shows that:
have profound effects on intestinal microbial composition, microbial functions, and interactions between the host’s immune system and gut microbiota. Consequently, the gut microbiota affects the absorption and metabolism of Tryptophan from the host and, directly or indirectly, regulates the subsequent physiological and immune responses of the host.
[/register]
Subscribe now to the technical magazine of animal nutrition
AUTHORS

Nutritional Interventions to Improve Fertility in Male Broiler Breeders
Edgar Oviedo
The Use of Organic Acids in Poultry: A Natural Path to Health and Productivity
M. Naeem
Synergistic Benefits of Prebiotics and Probiotics in Poultry, Swine, and Cattle
Gustavo Adolfo Quintana-Ospina
Hybrid Rye Potential in Laying Hen Feed Rations
Gwendolyn Jones
A day in the life of phosphorus in pigs: Part I
Rafael Duran Giménez-Rico
Use of enzymes in diets for ruminants
Braulio de la Calle Campos
Minerals and Hoof Health in the Pregnant Sow
Juan Gabriel Espino
Impact of Oxidized Fats on Swine Reproduction and Offspring
Maria Alejandra Perez Alvarado